Abstract
Multiple myeloma (MM) is an almost-always incurable malignancy of plasma cells. Chimeric antigen receptors (CARs) are artificial fusion proteins that incorporate antigen-recognition domains and T-cell signaling domains. CAR T cells have been shown to have substantial activity against leukemia and lymphoma, which has generated great interest in developing CAR T-cell therapies for MM. CAR T cells targeting B-cell maturation antigen (BCMA) have been shown to have substantial activity against MM (Ali et al. Blood. 2016. 128(13). 1688-1700). Improvements in CAR T-cell therapies for MM are needed. One possible limitation of CAR T-cell therapies is generation of recipient anti-CAR immune responses. Recipient anti-CAR immune responses have been reported in patients on clinical trials of anti-CD19 CARs (Turtle et al. Journal of Clinical Investigation. 2016. 126 (6). 2123-3138). Recipient anti-CAR immune responses could potentially develop against murine antibody variable region domains that are included in the single-chain variable fragments (scFvs) of most CARs. In addition, recipient anti-CAR immune responses could develop against the artificial linker domain in the scFv or against junctions between different CAR components.
We designed CARs with antigen-recognition domains of single fully-human heavy-chain-only variable domains (FHVH). The FHVH CARs do not contain a light chain variable region domain or a linker. The fully-human variable region, lack of a linker, and the minimized number of junctions should all lead to reduced immunogenicity for the FHVH antigen-recognition domain compared with a standard scFv.
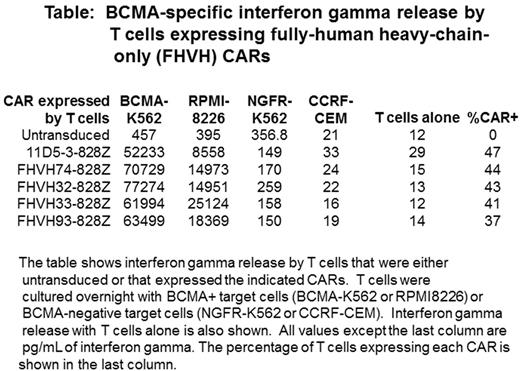
We constructed CARs with 4 different FHVH domains designated FHVH32, FHVH33, FHVH74, and FHVH93. We designed CARs with each of these FHVH domains along with a CD8-alpha hinge and transmembrane domain, either a CD28 or a 4-1BB costimulatory domain, and a CD3-zeta T-cell activation domain. DNA encoding these CARs was ligated into a gamma-retroviral vector, and primary human T cells were transduced with gamma-retroviruses encoding each of the CARs. We tested the T cells for CAR expression and a variety of T-cell functions. We assessed T-cell surface CAR expression by staining with phycoerythrin-labeled BCMA protein. T cells from 3 MM patients were transduced with genes encoding each of the CD28-containing CARs. The following mean percentages of T cells expressed each CAR after transduction: FHVH32-CD828Z (47.3%), FHVH33-CD828Z (49.6%), FHVH74-CD828Z (51.3%), and FHVH93-CD828Z (46.6%). The mean T-cell surface CAR expression of the 11D5-3-CD828Z CAR containing a standard murine scFv with a heavy chain variable domain and a light chain variable domain connected by a linker was 60.4%. Importantly, we found that each of the FHVH CARs specifically recognized BCMA in functional assays. Specific interferon gamma production by the CARs is shown in the Table. We also demonstrated BCMA-specific tumor-necrosis factor-alpha release, cytotoxicity, and proliferation by T cells expressing each of the 4 FHVH CARs containing CD28. We have previously reported clinical anti-myeloma responses with infusions of T cells expressing the 11D5-3-CD828Z CAR (Ali et al. Blood. 2016. 128(13). 1688-1700). T cells expressing FHVH CARs with CD28 domains, especially FHVH33-CD828Z and FHVH74-CD828Z, had BCMA-specific functions including cytokine release that were equal to or superior to those of T cells expressing 11D5-3-CD828Z (Table). We have demonstrated that human T cells expressing either FHVH33-CD828Z or FHVH74-CD828Z can eliminate established tumors of BCMA-expressing human RPMI8226 cells from immune-deficient mice.
We also assessed FHVH CARs with 4-1BB costimulatory domains. Of the 4-1BB CARs, FHVH33-CD8BBZ CAR had the highest percentage of T cells with surface CAR expression (mean 52.1%, n=3). T cells expressing FHVH33-CD8BBZ exhibited BCMA-specific cytokine-release, degranulation, cytotoxicity, and proliferation.
In summary, we have designed, constructed, and tested a novel type of CAR with an antigen-recognition domain made up of only single fully-human heavy-chain variable region domains. These CARs specifically recognized BCMA in vitro, and they eradicated tumors from mice. The simple, fully-human antigen-recognition domains in these CARs offer a potential advantage of decreased immunogenicity compared to other CAR designs.
Trinklein: TeneoBio: Employment. Buelow: TeneoBio: Employment. Kochenderfer: Bluebird bio: Research Funding; N/A: Patents & Royalties: I have multiple patents in the CAR field.; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal